Age-related macular degeneration (AMD)
AMD is the leading cause of blindness in the elderly population and efficient therapeutic options to treat the common form, dry AMD, are very limited. AMD is characterized by progressive degeneration of the central region of the retina, the macula. AMD patients lose their central visual field, making it impossible to read, write, drive and recognize faces, which greatly impedes an independent and active life. AMD is a very complex disease, caused by an interplay of diverse risk factors, including genetic predisposition, age and life-style.
Main Research Fields
Genetic and lifestyle risks driving AMD
Our research takes a systems biology approach combining molecular biology, bioanalytics and proteogenomics to uncover AMD disease mechanisms. We aim to develop precision medicine approaches to treat AMD based on the understanding of cellular communication networks.
Analyzing the impact of genetic risks for AMD, we focus on the complement system genes on chomosome 1, particularly the Complement factor H (CFH), and genes on chromosome 10q26, particularly HTRA1 and ARMS2. We analyze how these risk factors predispose to AMD, dependent on age and lifestyle of an individual. A comprehensive analysis of factors for AMD risk prediction has been carried out by the EU funded European Research cluster EYE-RISK (www.eyerisk.eu) with a focus on dry AMD, coordinated by Marius Ueffing. We continue our work within the German National Cohort NAKO (www.nako.de), currently analyzing AMD natural history of disease on a population-based level.
AMD research models
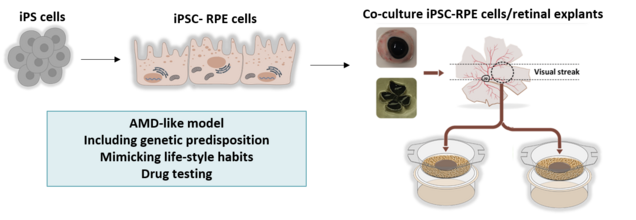
Our lab has developed AMD research models to better understand AMD pathology and to move forward in the discovery of potential therapeutic targets for AMD. Our organotypic models allow the analysis of complex cell-cell interactions within the retina, as well as the complex interplay of the risk factors for AMD. These include genetic risk and life-style habits, like diet or smoking but also factors and mechanisms protecting from AMD. For this purpose, we have created a novel model comprising human RPE-cells in co-culture with porcine retinal explants highly resembling the human macula. We use this model to investigate AMD risk factors in their impact on RPE cell- and neuroretinal function. We test and validate potential drugs to treat AMD using these models. In addition, we are employing AMD patient-derived iPSC-RPE cells to investigate the influence of different genetic risks on RPE physiology.
Current Projects
- Impact of Complement Factor H Y402H polymorphism on RPE cells and AMD pathology. University of Tübingen ƒortüne-programme (2021 – 2023)
- Elucidating the role of ARMS2 in extracellular matrix homeostasis and in choroidal neovascularization in AMD pathogenesis. Helmut-Ecker Foundation (2022-2024)
- Investigating CFH Y402H phenotype in Retinal Pigment Epithelium (RPE) cells and retina in a novel co-culture model of Age-related macular degeneration (AMD). DFG (2024-2026)
Collaborations
- Caroline Klaver, Erasmus Medical Center, Dept. of Ophthalmology and Epidemiology, Rotterdam, NL
- Alan Wright, Chloe Stanton, The MRC Human Genetics Unit at the University of Edinburgh, UK
- Berta De La Cerda Haynes, CABIMER (Andalusian Molecular Biology and Regenerative Medicine Centre), Sevilla, Spain
- Michela Deleidi, Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE), Tübingen, Germany
- Katja Schenke-Layland, Julia Marzi, Natural and Medical Sciences Institute at the University of Tübingen (NMI) Reutlingen/Tübingen, Germany
- Simon Clark, Institute for Ophthalmic Research, University of Tübingen, Germany
- Philipp Berens, Hertie Institute for AI in Brain Health, University of Tübingen, Germany
Selected Publications
- Merle DA, Sen M, Armento A, Stanton CM, Thee EF, Meester-Smoor MA, Kaiser M, Clark SJ, Klaver CCW, Keane PA, Wright AF, Ehrmann M, Ueffing M. 10q26 - The enigma in age-related macular degeneration. Prog Retin Eye Res. 2022 Dec 10:101154.
- Thee EF, Colijn JM, Cougnard-Grégoire A, Meester-Smoor MA, Verzijden T, Hoyng CB, Fauser S, Hense HW, Silva R, Creuzot-Garcher C, Ueffing M, Delcourt C, den Hollander AI, Klaver CCW. The phenotypic course of age-related macular degeneration for ARMS2/HTRA1: The EYE-RISK Consortium. Ophthalmology. 2022 Jul;129(7):752-764.
- Merle DA, Provenzano F, Jarboui MA, Kilger E, Clark SJ, Deleidi M, Armento A. and Ueffing M.. mTOR inhibition via Rapamycin treatment partially reverts the deficit in energy metabolism caused by FH loss in RPE cells. Antioxidants. 2021.
- Armento A, Murali A, Marzi J, Arango-Gonzalez B, Kilger E, Clark SJ, et al. FH loss in RPE cells causes retinal degeneration in a human RPE-porcine retinal explant co-culture model. Biomolecules. 2021;11(11):1621.
- Armento A, Schmidt TL, Sonntag I, Merle D, Jarboui MA, Kilger E, et al. CFH loss in human RPE cells leads to inflammation and complement system dysregulation via the NF-κB pathway. IJMS 2021.
- Armento A, Ueffing M, Clark SJ. The complement system in age-related macular degeneration. Cell Mol Life Sci. 2021;78(10):4487-505.
- Colijn JM, Meester-Smoor M, Verzijden T, de Breuk A, Silva R, Merle BMJ, Cougnard-Grégoire A, Hoyng CB, Fauser S, Coolen A, Creuzot-Garcher C, Hense HW, Ueffing M, Delcourt C, den Hollander AI, Klaver CCW; EYE-RISK Consortium. Genetic Risk, Lifestyle, and Age-Related Macular Degeneration in Europe: The EYE-RISK Consortium. Ophthalmology. 2021 Jul;128(7):1039-1049.
- Ajana S, Cougnard-Grégoire A, Colijn JM, Merle BMJ, Verzijden T, de Jong PTVM, Hofman A, Vingerling JR, Hejblum BP, Korobelnik JF, Meester-Smoor MA, Ueffing M, Jacqmin-Gadda H, Klaver CCW, Delcourt C; EYE-RISK Consortium. Predicting Progression to Advanced Age-Related Macular Degeneration from Clinical, Genetic, and Lifestyle Factors Using Machine Learning. Ophthalmology. 2021 Apr;128(4):587-597.
- Armento A, Honisch S, Panagiotakopoulou V, Sonntag I, Jacob A, Bolz S, et al. Loss of Complement Factor H impairs antioxidant capacity and energy metabolism of human RPE cells. Sci Rep. 2020;10(1):10320.
- Handa JT, Bowes Rickman C, Dick AD, Gorin MB, Miller JW, Toth CA, Ueffing M, Zarbin M, Farrer LA. A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration. Nat Commun. 2019 Jul 26;10(1):3347.
- Kortvely E, Ueffing M. Common mechanisms for separate maculopathies? Adv Exp Med Biol. 2012;723:61-6.
- Stanton CM, Yates JR, den Hollander AI, Seddon JM, Swaroop A, Stambolian D, Fauser S, Hoyng C, Yu Y, Atsuhiro K, Branham K, Othman M, Chen W, Kortvely E, Chalmers K, Hayward C, Moore AT, Dhillon B, Ueffing M, Wright AF. Complement factor D in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011 Nov 11;52(12):8828-34.
- Kortvely E, Hauck SM, Duetsch G, Gloeckner CJ, Kremmer E, Alge-Priglinger CS, Deeg CA, Ueffing M. ARMS2 is a constituent of the extracellular matrix providing a link between familial and sporadic age-related macular degenerations. Invest Ophthalmol Vis Sci. 2010 Jan;51(1):79-88